After messing up with Lewis dot structures on a chemistry test a while back (I forgot to draw any of the dots. Otherwise it would have been nearly 100% 🙁 ), so I wanted to come up with an alternate to Lewis dot structures that doesn’t need dots. I still haven’t figured out a way to do that, but at the time I instead ended up with a way to format a Lewis dot structure in a way that you could type it. Note: you must already know how to draw out simple Lewis dot structures and the terminology behind them to understand this post.
Here’s a table to represent the various symbols that you use to type the Lewis dot structures
The symbol for (a)…
- Single bond is a dash (-)
- Double bond is an equal sign (=)
- Triple bond is an equal sign and a dash (=- or -=)
- Quadruple bond is two equal signs (==)
- (If you need greater values, combine the dashes and equal signs until you have the number you need)
- One valence electron is a period (.)
- Two valence electrons is a colon (:)
- Three valence electrons is a colon and a period (.: or :.)
- Four valence electrons is two colons (::)
- (If you need greater values, combine the periods and colons until you have the number you need)
To help with typing the Lewis dot structures, parenthesis will need to be used around each atom in the molecule. The first set is parenthesis (), the second set is brackets [], the third set is braces {}, and if you need more than either loop back to parentheses or don’t bother typing it. For example, Nitrogen’s Lewis dot structure would be (N:.), with the colon+period representing nitrogen’s three valence electrons. O2’s Lewis dot structure would be [::O=(O::)].
There are two colons next to each O to represent the four valence electrons that each oxygen has, and the equal sign to represent the double bond. Why one atom is in parenthesis and the other is not will be explained next.
A more complicated example would be CH4’s typed Lewis dot structure: [C-(H)-(H)-(H)-(H)]
None of the atoms in this molecule have any valence electrons, so there aren’t any periods or colons. Something more noticeable about it is that the hydrogen atoms, while seemingly connected in the typed version, aren’t connected in the drawn version. The way you get around that is by having the atom that is being connected to outside of the parentheses. The carbon atom has only the brackets around it, meanwhile all of the hydrogen atoms also have the parentheses.
You can even type the Lewis dot structure of a molecule by using all three bracket types, such as C2H3N’s molecule: {C-[H]-[H]-[H]-[C-=(N:.)]} .
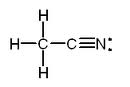
The typed Lewis dot structure starts with the left carbon, showing it with only the braces surrounding it. That means that all of the atoms in the molecule that are only surrounded by both the braces and the brackets are connected to the carbon atom. Any atoms that are also surrounded by parentheses aren’t connected to the first carbon. The second carbon is surrounded by both the braces and the brackets, but not the parentheses. That means that any molecules that are surrounded by the braces, the same pair brackets that the 2nd carbon is surrounded by, and parentheses are connected to it. The nitrogen in the molecule fits all of those criteria, and as you can see in the drawn out version it is connected to the 2nd carbon but not the first.
Unfortunately this form obviously has limitations, such as those that a normal Lewis dot structure has, as well as the fact that the typed diagrams will rapidly get very complex.
Something I’m experimenting with is how to type the structure when it has rings in it, like carbon rings.
One possible way is to use something to communicate a linked section of a sort, like asterisks do for footnotes. So the above’s typed Lewis dot structure, starting with the bottom left carbon and going around clockwise, would be {C-[*]-[H]=[C-(H)-(C-{H}={C-[H]-[*C-(H)]})]}
For fun, and also to test this method of diagramming molecules, I Googled “Large Lewis dot structures” to see what came up, and decided that I would type it out myself at some point. Until I find the time to do what is probably going to be a long and difficult task, here’s the molecule I found:

Also, I’m thinking that there is some way to show the molecular polarity (or maybe the proper term is electronegativity) using greater than and less than signs (<>), but until I find my Chemistry book I can’t remember any of the proper terms, so until then just know that there’s more to come.
~ George